In the rankine cycle, water is first pressurized and then heated to produce steam before it is used to turn a turbine. As far as i know, this is what is considered the best way to extract mechanical work on the turbine, but i want to understand why this is so. How is it that we can extract more work on a turbine from steam than from regular unheated, un pressurized water?
1 Answers
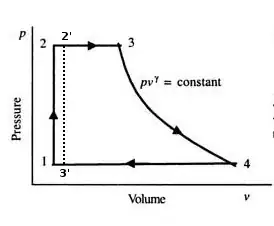
A good way to understand why the phase change is so important is by looking at the Pressure vs. Volume diagram for the Rankine cycle (path 1-2-3-4-1):
Remember that the work output of any thermodynamic cycle is the integral of its P-V diagram (area under the curve). If the water in the Rankine cycle were not heated to steam, its change in volume would be very small, both in the pumping/heating processes and in the expansion/cooling processes. And, if its volume didn't change significantly, its P-V curve would have almost no area. Thus, the cycle would produce much less work output than if the volume changed significantly and produced a P-V diagram with a large cyclic integral. The dashed line (path 1-2-2'-3'-1) represents what the path might look like if plain water were used.
Many early heat engines used a gas-phase-only working fluid; it was not until Rankine or DeLaval came along (can't remember who), and discovered the benefits of using a phase change in the cycle.
- 3,064
- 2
- 15
- 28