I agree with Chemodynamics, and I will try to add a different perspective (or maybe two).
The fire process is a process where you add a fuel (i.e. energy in the system), and that fuel is gradually consumed. The rate at which it is consumed releases the chemical energy. An interesting thing here is that:
- the log is a three dimensional object
- the fire will only occur on the external surfaces of the wood.
The above reason is why, if you put small pieces (or shredded) of wood in a fire (because the surface area is larger) the fire seems to grow stronger much faster and the flame is hotter (compared to a log). What happens then is that the flame releases more energy in the unit of time, which heats up the surrounding material (providing the required activation energy) and sustains the combustion.
Perspective No 1:
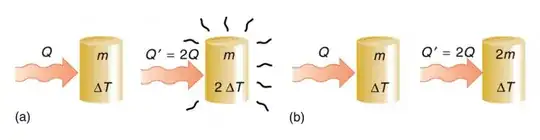
The energy released by the flame is governed by a square law of the dimensions of the object, while the thermal capacity is governed by the mass (which follows a cube law wrt the dimensions of the object).
At this point its noteworthy that in order to understand fully a combustion process you need to be familiar with concepts like internal energy, enthaphy, specific work due to volumetric changes and others. However, I will try to explain it a more descriptive manner.
The flame of a self sustaining combustion process releases at least enough energy to heat up the surrounding material just enough to be able to ignite (this is called activation energy). A rest of the remaining energy raises a) the temperature of the fuel (and oxidizing agent) even further, and also b) it heats up the environment (which is the useful part).
What you feel as heat when you burn a log is either convective or radiative. The convective heat transfer has to do with the movement of combustion gases (and it increases with the speed of the gases and also the temperature of the gases). The radiative is only depended on the temperature and increases by a 4th order of the temperature. That means that doubling the temperature (in Kelvin) results in 16 times increase in radiative heat losses to the environment.
So assuming 300 K is the ambient temperature, then at 1823 °C (or approx 2100 K), the radiative losses would increase by an order of 74(= 2400).
Perspective No 2:
As the temperature of a flame increases the radiative losses to the environment increase with a power law (4th order). So, despite adding more logs to the pile, once the temperatures reaches a high, the radiative losses are equal to the energy released and there is not enough energy to heat up more the remaining mass in the fire.
Both from logic and from experience, I suggest temperature is measurably increased by putting more logs on the fire.
– Robbie Goodwin Jun 08 '21 at 02:27