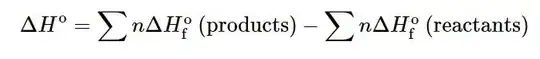Standard enthalpy of formation is when one mole of compound is formed from its constituent elements.
Example : $H_2$(g) + $O_2$(g) —>$ H_2O$(L).
But we don’t say the same for $CaO + CO_2$ —> $CaCO_3$.
It is because $CaCO_3$ is not formed it’s constituent elements but from other compounds.
Q1: From other compounds , does it mean the most simplest state I.e it is not formed from Ca + $O_2$.
I want to confirm if that’s all the meaning of standard enthalpy of formation. I am getting a lot confused over it.
Q2 Also , for the equation above. We can never find standard enthalpy of formation but can find the standard enthalpy of reaction I.e addition of $\delta$H of $CaO $ in solid state + $CO_2$ in gaseous state ?