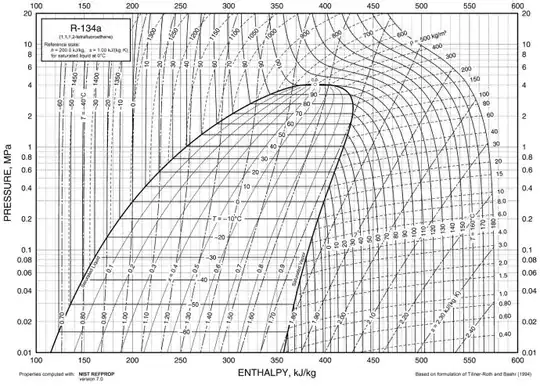
How do I find the entropies for T1 = -10 deg C and T2 = 30 deg C, respectively using the R-134a refrigerant chart (SI):
1 Answers
Instead of giving you fish I will teach you how to catch it.
There isn't a particular entropy at a given temperature. Considering the wet region there should be given a dryness fraction value for the entropy. With dryness fraction value and temperature combined, you can calculate the entropy of the refrigerant:
$$entropy (s) = sf + x * sfg$$
Where:
$\text{sf = the entropy of saturated liquid R-134a}$
$\text{x = the dryness fraction (between 0 and 1)}$
$\text{sfg = the entropy change during the evaporation process}$
Now for your question:
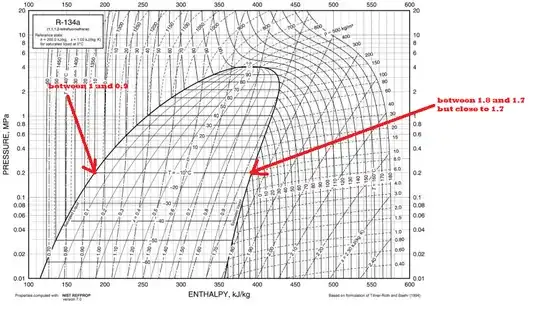
See the image below. The sf value can be taken from the chart. See the left value. It's between 1 and .9.
Now see the (sfg + sf) value on the right. When you equate it with a certain dryness fraction x you will get entropy at that particular point.