How is it possible for silicon to be used in many engineering materials like oil (Silicone oil), grease, caulk, rubber(PDMS), resin? What property of silicon allows it to be so used?
3 Answers
Silicon forms bonds in a similar way to carbon, and has a similar outer-shell electron orbital configuration. Carbon, as you may well know, has the ability to covalently bond with up to four lone pairs of electrons on neighboring atoms in a generally tetrahedral arrangement, giving carbon a large versatility in molecular configuration and conformation. The versatility of carbon allows for a wide range of biomolecules, polymers, and other complex organic molecules. Silicon has a similar bonding arrangement, i.e. tetrahedral, except that silicon-silicon bonds are less strong and less flexible than carbon-carbon bonds, and silicon naturally tends to form the highly stable diamond silicon lattice. The diamond silicon lattice is brittle and semi-conducting, but not terribly useful for polymeric materials. Indeed, I have not heard of successful attempts to form economical, purely silicon-based polymers.
However, as you noted, most silicon-based polymer materials are silicone, or perhaps more accurately polysiloxane. Polysiloxanes are materials which contain a repeating, alternating configuration of silicon and oxygen atoms along the primary backbone. Polysiloxanes are generally mechanically flexible molecules which retain the configurational and conformational advantages of the tetrahedral bonding of silicon.
- 4,079
- 1
- 18
- 32
Starrise gave an answer describing silicone from an engineering/chemical perspective, but I disagree that this is the reason for silicone to be so widely used.
The real reasons why it is used so widely:
It is versatile. It is used as a grease (like other greases, e.g. petroleum based ones) because it provides good lubrication. It is used as a caulk (like other caulks, e.g. cement or acrylic based caulks) because it makes a good sealant and can be squeezed between two tiles and can cure. It is used as in rubbers, because it has the necessary qualities like being flexible and insulating.
It is inert. This is extremely important! Organic materials are rarely durable, but even heavily processed organic-based synthetics or inorganic ones tend to change a lot over time. Fats go rancid, plastics become brittle, petroleum based greases can get quite thick over time. Silicone doesn't change much with heat, UV light, time, or contact with oxygen, and is also quite resistant to many chemicals which would stain, corrode or otherwise damage a material.
It has an extremely low toxicity (at least the finished product, I don't know about the production process). You can bake in it, smear it on your hair, drink it, or implant it in your body, and everything goes well.
The raw materials are plentiful, literally "like sand at the sea" as the German proverb goes. No need to worry about sourcing rare elements, or the declining oil reserves. No bad association with "you are killing our nature by mining/deforesting/whatever".
It makes user friendly products which are both useful and attractive. If you make a caulk out of it, it cures after air exposure, without stinky fumes. If you sell it as a baking form, it releases the cake without fuss. If you make a lubricant out of it, it doesn't have to smell or look a dirty brown. It takes colors well, is easily shaped, has a clean pleasant feel when touched, does not smell, easy to clean, somewhat water repellent, does not tear too easily, does not wear out too much.
Of course, the atomic structure described by the other answer is, in a sense, the reason why silicone happens to have the qualities described here. But while it is further back in the chain of causality, it is not really as important for the question you asked. Because, if another atomic structure had produced a material of equal utility and marketability, we would have used it just as happily (and do so, for other applications where slightly different qualities are needed).
- 180
- 4
The key characteristic of silicone polymers that make it so versatile is that they are not a single compound. Just like polyurethanes are extremely versatile organic polymers, polysiloxanes are extremely versatile organic/inorganic polymers.
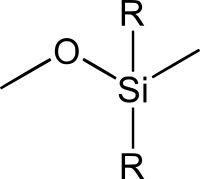
The backbone of the polysiloxane alternates silicon and oxygen atoms and as such is inorganic. But much of what gives this family of molecules its versatility is the variety of organic compounds on the Si atoms in the backbone:
Siloxane by Sei. CC BY-SA 3.0
Here the R groups can be a wide variety of organic group. If both R groups are methyl you get the vary famous polydimethylsiloxane. Cross-linkers can also be included. These can be sensitive to humidity so that they cure at room temperature (caulk) or fancier systems that cure in the lab to produce silicone rubbers.
So the answer to your question is that silicones are not a single substance that is super versatile, but a family of polymers that can vary in chemistry and structure, providing a wide range of controllable properties. Their main advantage over purely organic polymers are well listed in @rumtscho's answer.
- 508
- 3
- 13